

In electric cars, energy undergoes the following conversions: from stored chemical energy to electrical energy and from electrical energy to mechanical energy. Sure electric vehicles have an efficiency of about 70%, but in this case, we are not talking about thermal efficiency because an electrical motor is not a heat engine. In these cases, comparisons are often made for incomparable characteristics. Sometimes you can find comparisons between combustion engines and electric motors or conventional power plants and solar power plants. In modern nuclear power plants, the overall thermal efficiency is about one-third (33%), so 3000 MWth of thermal power from the fission reaction is needed to generate 1000 MWe of electrical power.Sub-critical fossil fuel power plants operated under critical pressure (i.e., lower than 22.1 MPa) can achieve 36–40% efficiency.In general, engines using the Diesel cycle are usually more efficient. A typical diesel automotive engine operates at around 30% to 35%.About 70-75% is rejected as waste heat without being converted into useful work, i.e., work delivered to wheels. A typical gasoline automotive engine operates at around 25% to 30% of thermal efficiency.What are the typical thermal efficiencies of heat engines? This inefficiency can be attributed to three causes. In modern nuclear power plants, the overall thermodynamic efficiency is about one-third (33%), so 3000 MWth of thermal power from the fission reaction is needed to generate 1000 MWe of electrical power. In a natural thermodynamic process, the sum of the entropies of the interacting thermodynamic systems increases.Īll conventional thermal power plants are heat engines subject to the efficiency limitations imposed by the second law of thermodynamics.Ī typical gasoline automotive engine operates at around 25% to 30% of thermal efficiency. The entropy of any isolated system never decreases. “It is impossible to construct a device which operates on a cycle and produces no other effect than the production of work and the transfer of heat from a single body”. “It is impossible to construct a device which operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body”. Listed below are three that are often encountered. The second law of thermodynamics may be expressed in many specific ways. Key Factsīefore these statements, we have to remind Carnot’s rule that specifies limits on the maximum efficiency any heat engine can obtain.
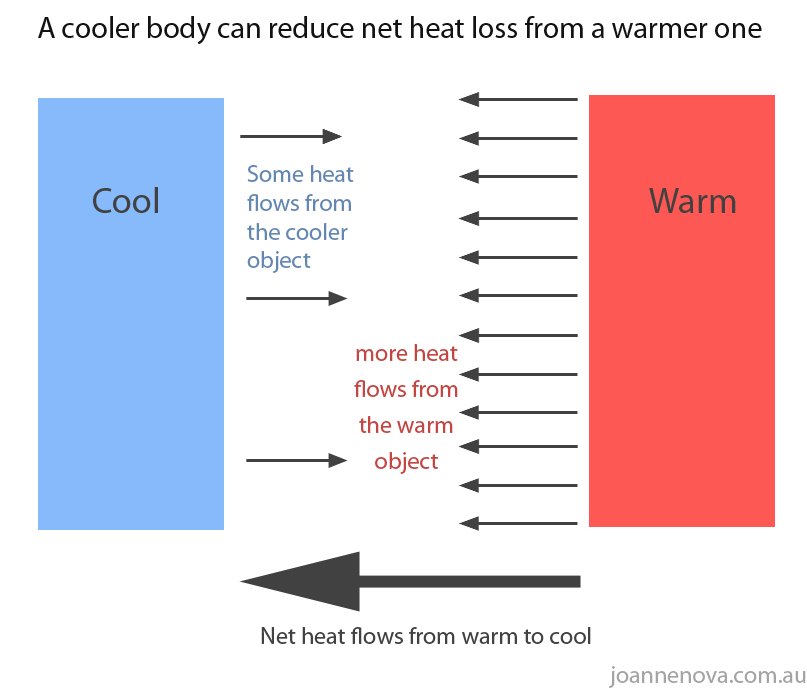
In a natural thermodynamic process, the sum of the entropies of the interacting thermodynamic systems increases. Article Summary & FAQs What states the second law of thermodynamics?Īccording to the second law of thermodynamics:


 0 kommentar(er)
0 kommentar(er)
